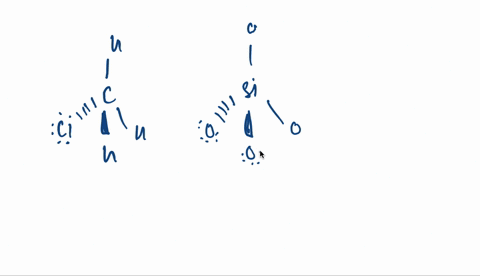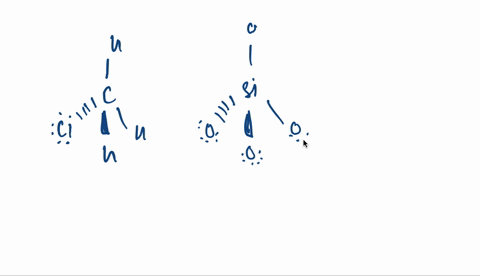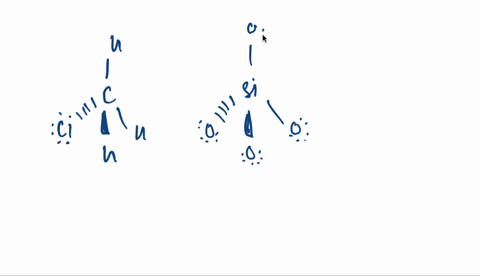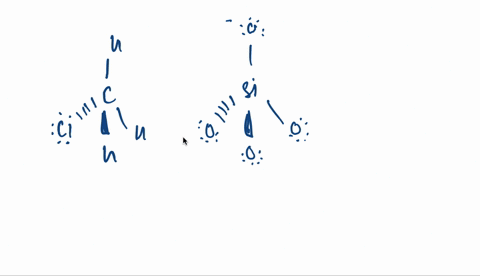Kapitel 3; Kovalent binding - Opgaver-kopi.docx - 18/07-2020 Basiskemi C Kapitel 3: Kovalent binding Kapitel 3: Kovalent binding Opgaver 29. Angiv navne | Course Hero

Kapitel 3; Kovalent binding - Opgaver-kopi.docx - 18/07-2020 Basiskemi C Kapitel 3: Kovalent binding Kapitel 3: Kovalent binding Opgaver 29. Angiv navne | Course Hero
Luminescent cyclometalated alkynylplatinum(ii) complexes with 1,3-di(pyrimidin-2-yl)benzene ligands: synthesis, electrochemistry