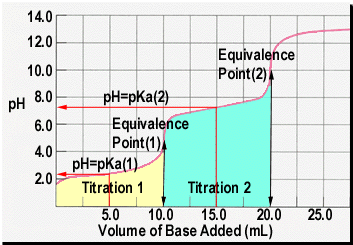
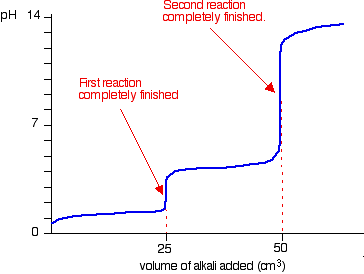
![Figure 2. pH curve of malonic acid (0.05 M) against standardized NaOH(0.09M) [pKa1=2.85, pKa2= 5.70] : Multiproticity of Weak Acids: Inflection Point vs. Equivalence Point : Science and Education Publishing Figure 2. pH curve of malonic acid (0.05 M) against standardized NaOH(0.09M) [pKa1=2.85, pKa2= 5.70] : Multiproticity of Weak Acids: Inflection Point vs. Equivalence Point : Science and Education Publishing](http://pubs.sciepub.com/wjce/4/1/4/bigimage/fig2.png)
Figure 2. pH curve of malonic acid (0.05 M) against standardized NaOH(0.09M) [pKa1=2.85, pKa2= 5.70] : Multiproticity of Weak Acids: Inflection Point vs. Equivalence Point : Science and Education Publishing

The current-pH curve for electrooxidation of Food Red 17 at a surface... | Download Scientific Diagram

ph - Why is the gradient of the curve of a strong base titrated with strong acid small up until equivalence? - Chemistry Stack Exchange

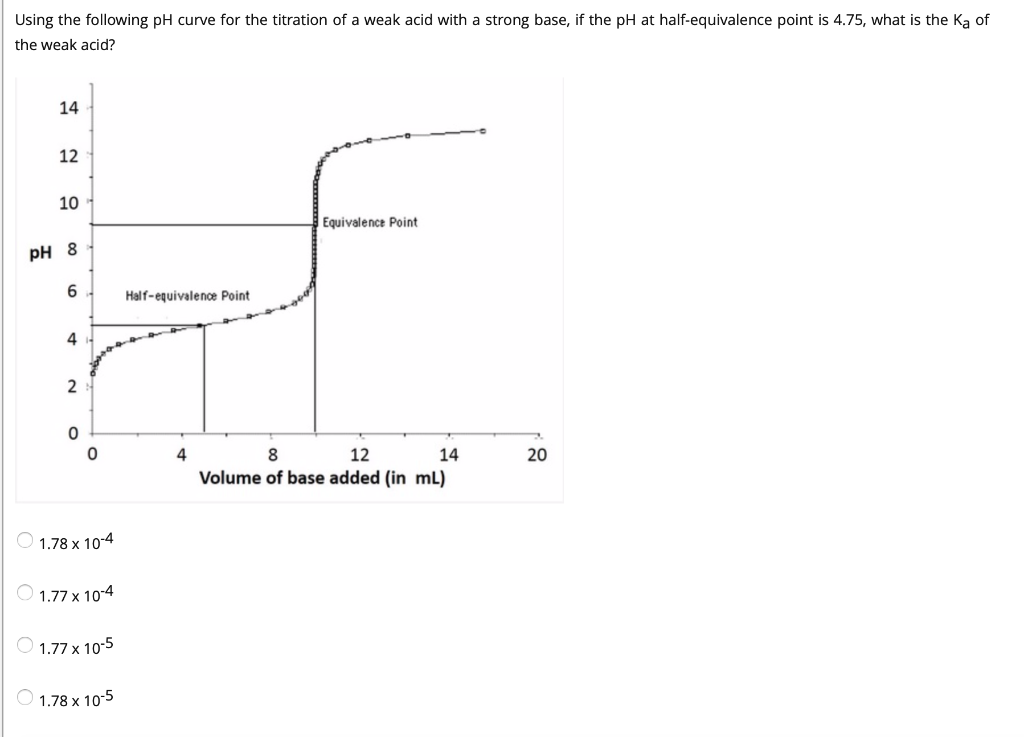
Below is the pH curve of a weak acid (HA) titrated with strong base. Answer the following questions based on the interpretation of this pH curve. a) What is the pH at
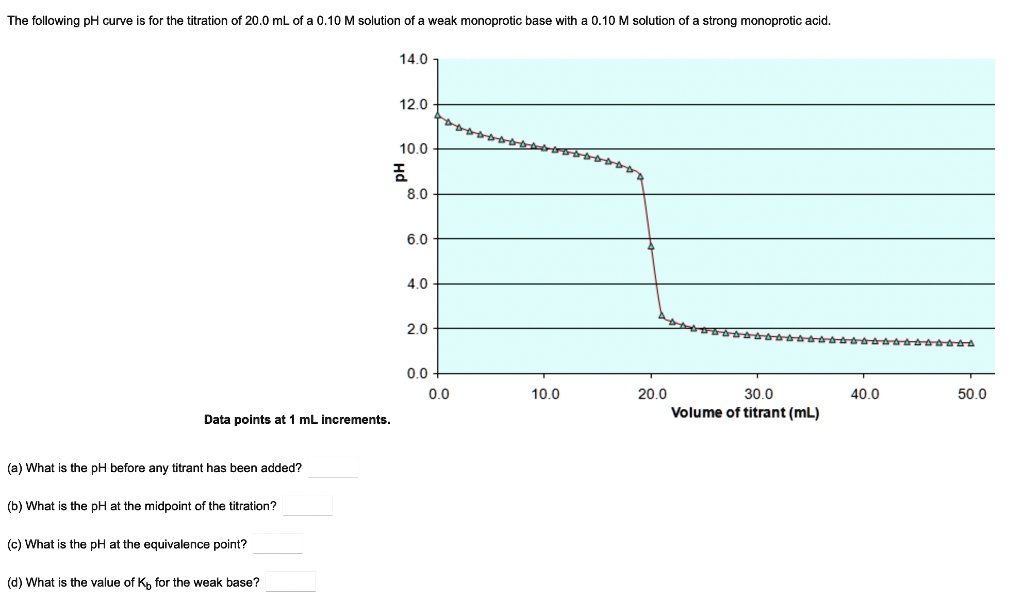
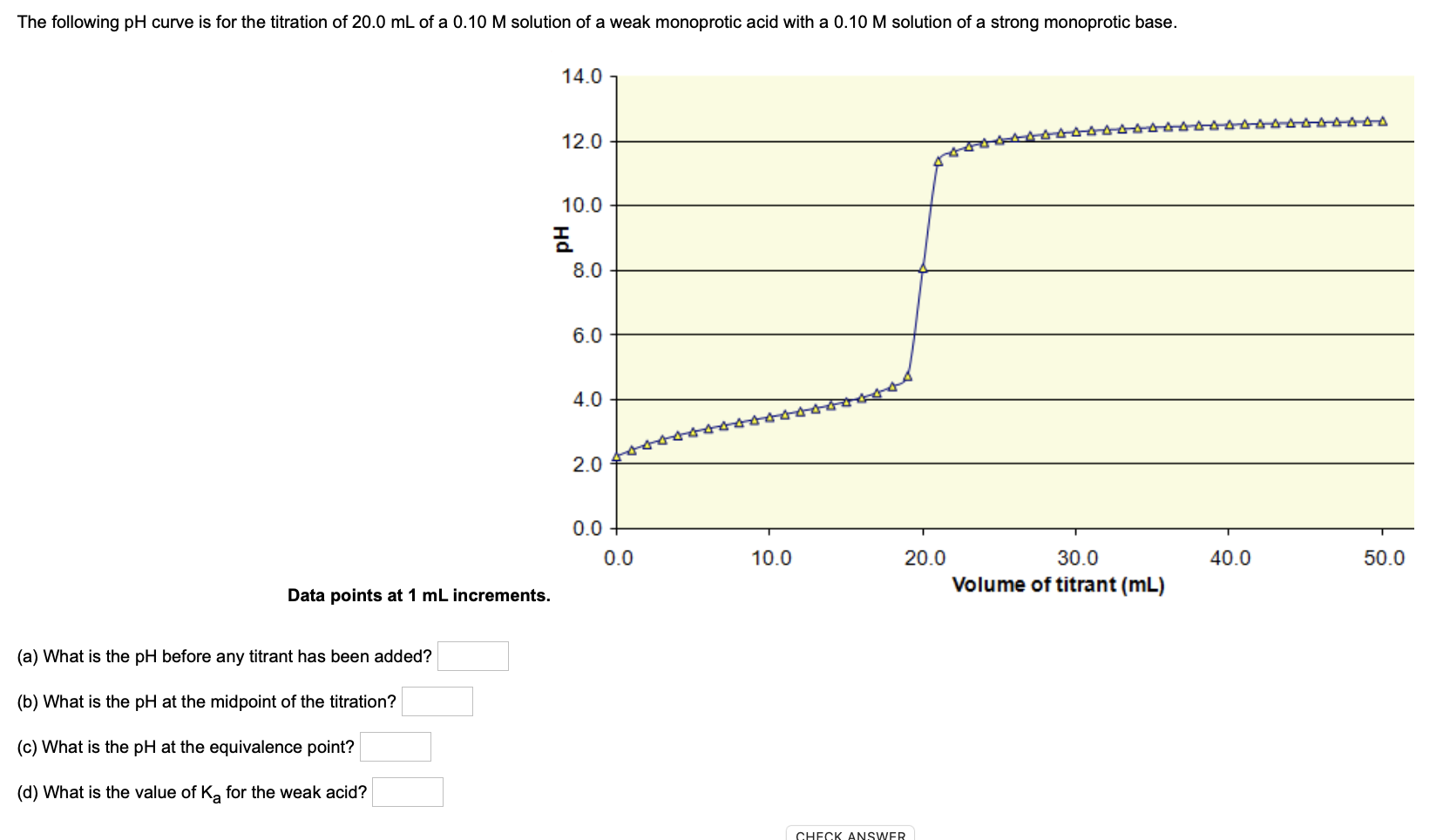
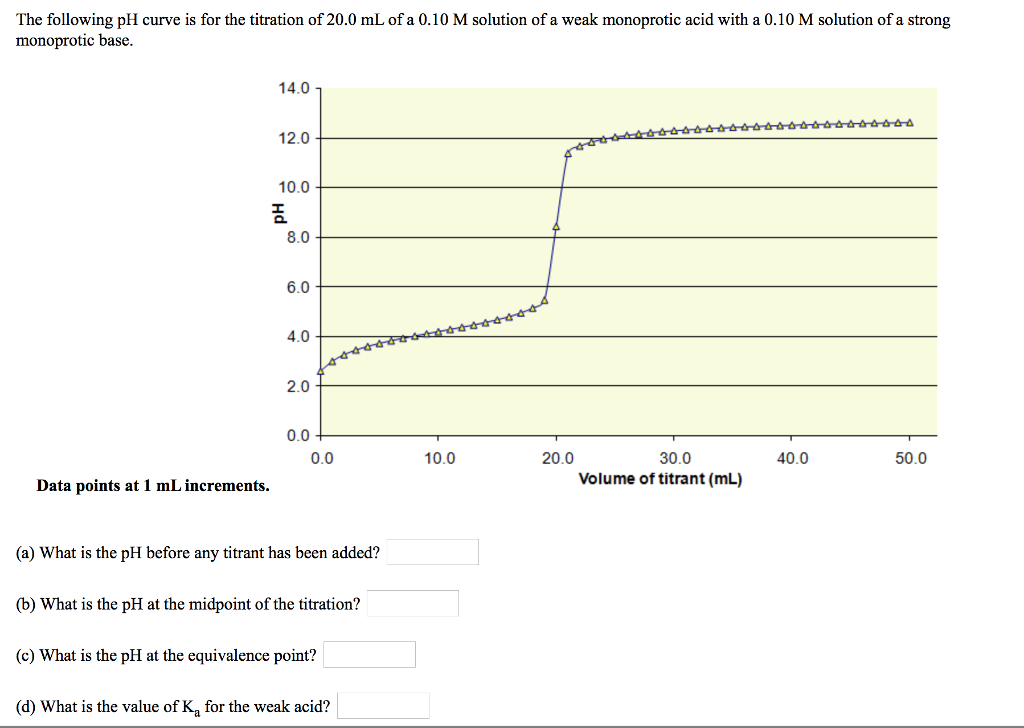
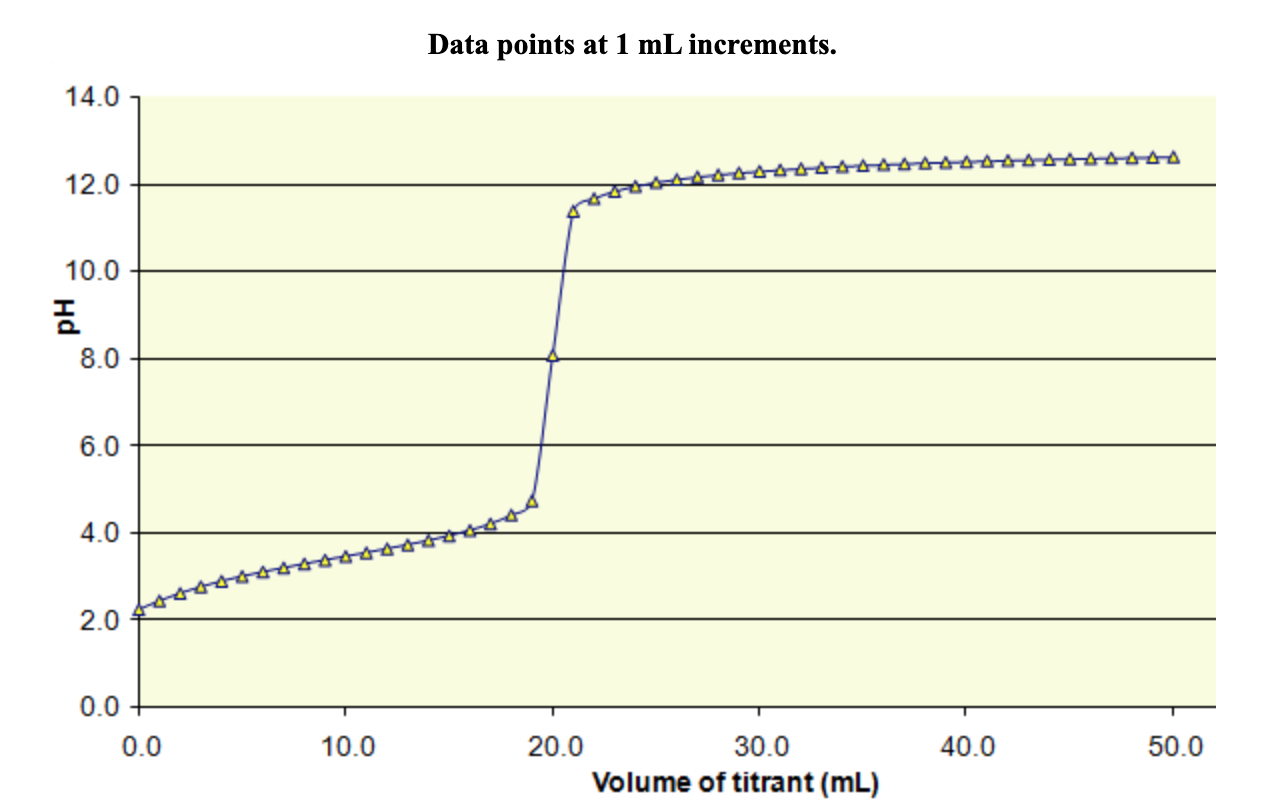
SOLVED: The following pH curve is for the titration of 20.0 mL of a 0.10 M solution of a weak monoprotic base with 0.10 M solution of strong monoprotic acid: 14.0 12.0