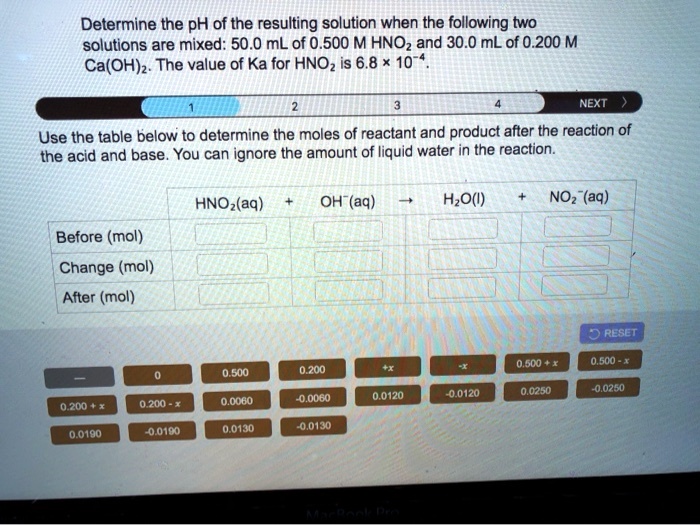
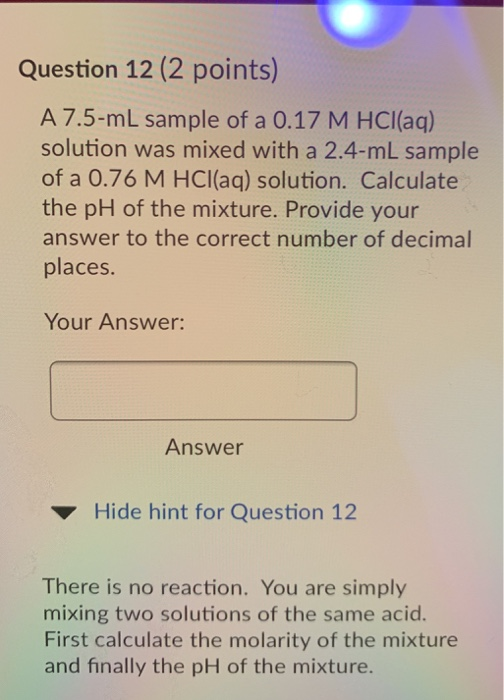
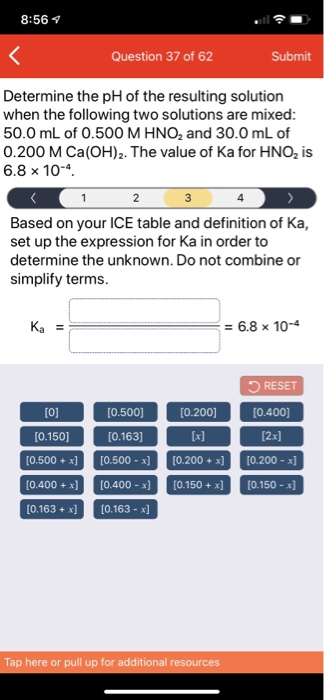
Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively.

Equal volumes of two solutions having pH = 1 and pH = 4 are mixed together. The pH of the resulting solution is:

What will be the resultant pH when 200mL of an aqueous solution of HCl (pH = 2) is mixed with 300mL of an aqueous solution of NaOH (pH = 12)?

Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively.

Calculate the pH of a solution formed my mixing equal volumes of two solutions A and B of a strong acid having pH=6 and pH=4 respectively.

Calculate the ph of a solution formed by mixing equal volumes of two solutions A and B of a strong acids having ph=6" and "ph=4 respectively.
Calculate the pH of a solution formed by mixing equal volumes of two solutions A and B of a strong acid having pH = 6 and pH = 4 respectively. - Sarthaks