Cephalon sales rep tells court Actiq, Fentora opioids were not viable sellers in W.Va. | West Virginia Record
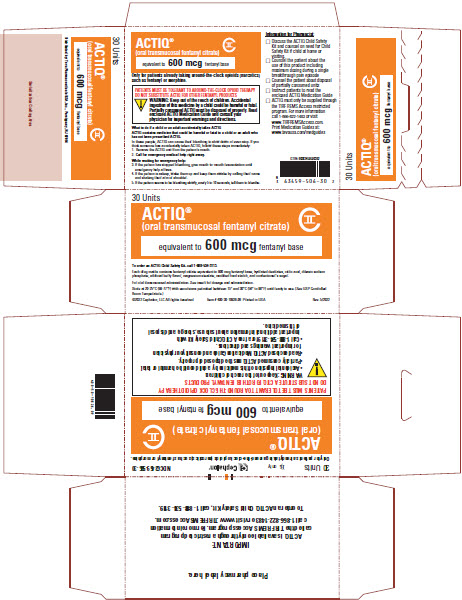
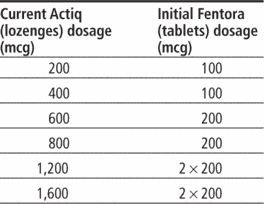
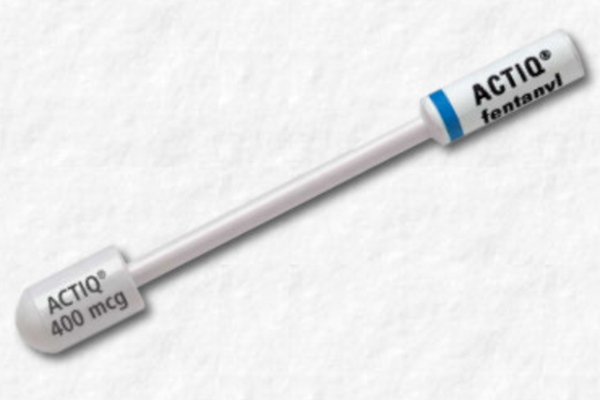
PRIOR AUTHORIZATION POLICY Opioids – Fentanyl Transmucosal Drugs Prior Authorization Policy • Abstral ® (fentanyl sublingua

Teva Pharmaceutical will pay over $4 billion in opioid settlement in case heard in Cleveland | Crain's Cleveland Business

Attorney General Josh Stein Announces $4.5 Billion Settlement in Principle with Opioids Maker Teva - NCDOJ
NYSDFS Enforcement Action - August 18, 2020: Statement of Charges and Notice of Hearing regarding Teva Pharmaceutical Industries

Teva Pharmaceutical Industries Limited (TEVA) - Pharmaceuticals & Healthcare - Deals and Alliances Profile | PDF | Initial Public Offering | Pharmaceutical Industry

Israeli drug company Teva reaches $4.3 billion settlement in US opioid lawsuits | The Times of Israel